Continuous Polymer Fractionation
Macromolecules are – unlike low molecular weight compounds - normally not uniform with respect to a number of aspects. In the first place this is the molar mass (mixtures of short and long chains) but also in the molecular architecture (e.g. linear or branched) and chemical constitution (e.g. different fraction of monomers). For basic science and for many practical applications this feature constitutes a big problem. The only way out of the difficulty consists in the fractionation of the materials. Several analytical methods can be used for that purpose; however, the preparation of reasonable amounts (which means at least on the order of 100 g) requires special techniques. Over the years we have developed several variants of continuous polymer fractionation processes as described below.
The basic principle of these methods consists in the establishment of liquid/liquid phase equilibria. Due to the larger attainable entropy of mixing, the lower molecular weight components of the starting material accumulate in the dilute coexisting phase (sol), while the higher molecular weight material is preferentially incorporated into the concentrated phase (gel), because of the enthalpically more favorable environment. The following chart demonstrates the situation for solutions of a polymolecular mixture of polystyrene in the marginal solvent cyclohexane. The dashed lines of the phase diagram represent coexistence curves. The molecular weight distributions of the fractions are shown on the right side. The red point designates the critical composition of the system.
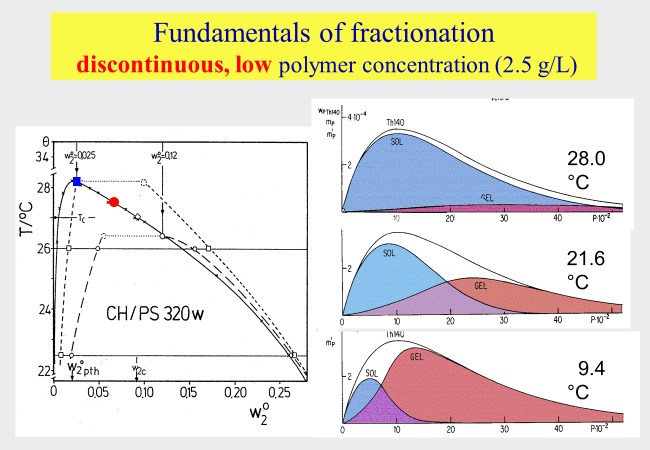
In the example shown above the polymer concentration is rather low and the temperature decides on the fraction of the material that is accumulated in the coexisting phases and governs the molecular weight distribution. The former feature hampers the production of larger amounts of material and the latter is experimentally very inconvenient.
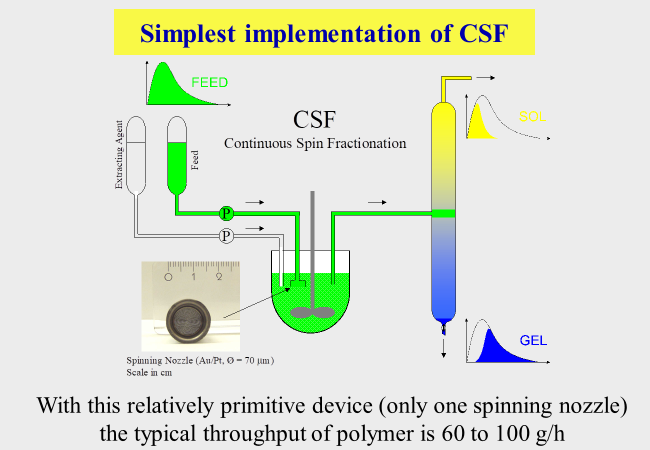
For that reason we use mixed solvents instead of single solvents, enabling the adjustment of solvent quality by means of the mixing ratio; furthermore the fractionation is run continuously as depicted below. The polymer material - initially only contained in the feed phase - is partially extracted by the mixed solvent and leaves the apparatus as gel, whereas the solvent - now loaded with polymer - leaves as the sol phase.

One of the problems with polymer fractionation consists in the fact that the efficiency would be largest with dilute systems, which however means the necessity to use huge amounts of solvent. Increasing the polymer concentration does not help too much because of the kinetically hindered mass transfer from the feed phase to the sol phase. Furthermore it lies in the nature of the separation mechanism that sharp cuts through the molecular weight distribution are impossible and even if they were, the obtained fractions would still be molecularly very non-uniform. There are two measures to solve the just described problem. The first one lies in the use of spinning nozzles to promote the transfer of material over the phase boarder by increasing the interfacial tension as illustrated below.
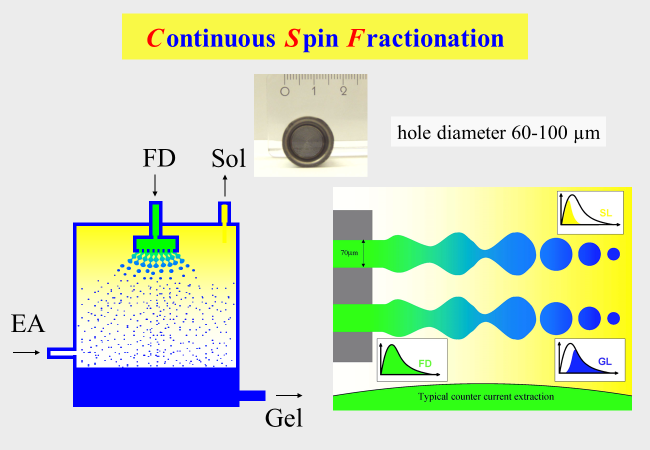
The second provision to accumulate sufficient amounts of material lies in a continuous process management. The next chart sketches the situation with CSF (Continuous Spin Fractionation). The solution of the unfractionated polymer (feed) is depicted in green, that of the unloaded solvent (extracting agent) is shown colorless. In the course of the extraction process only the long chains remain in the source phase, which leaves the apparatus as the gel phase (blue). The extracting agent accumulates the short chains and leave the apparatus as sol phase (yellow). To reach the best possible fractionation efficiency it is important to select the feed entrance into the separation column adequately to obtain the best fractionation as indicated below.
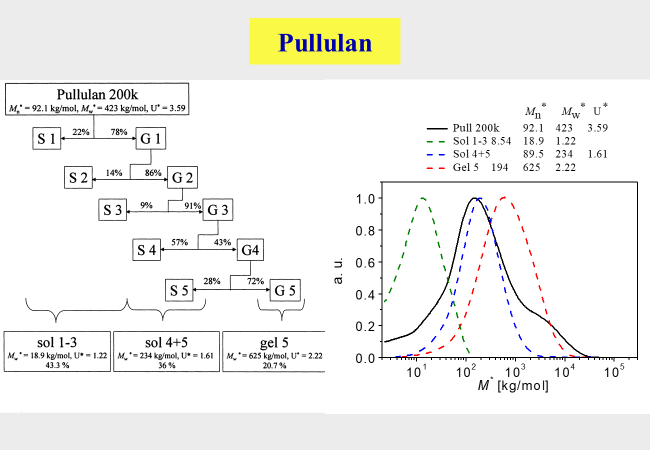
All the provisions described above do only in special cases yield the desired material in one step. One such example consists in the removal of harmful components at the ends of the initial molecular weight distribution. The inherent limitation in fractionation efficiency (cf. first chart of this section) requires several consecutive fractionation steps. The best manner to cope with that problem consists in the use of the gel fractions obtained in one CSF run directly as the feed for the next separation step. The following example describes the actual procedure for pullulan[^265], where the solvent was water and the precipitant acetone.
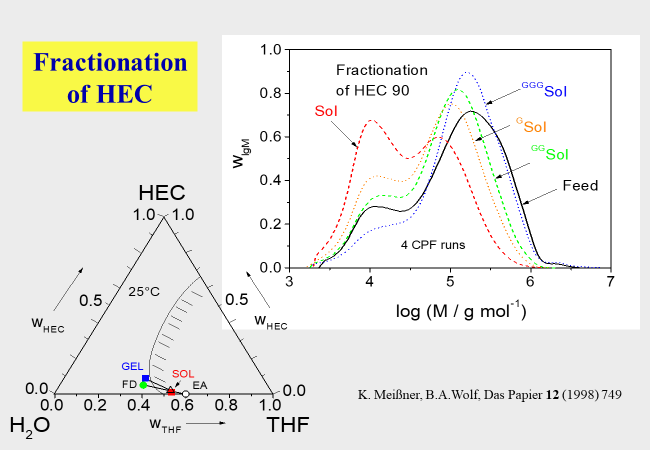
The fractionation of hydroxy ethyl cellulose[^227] is another example for the preparation of suitable material with desired molecular uniformity by the combination of several CSF steps; in this case the solvent was methyl acetate and the non-solvent 2-propanol.
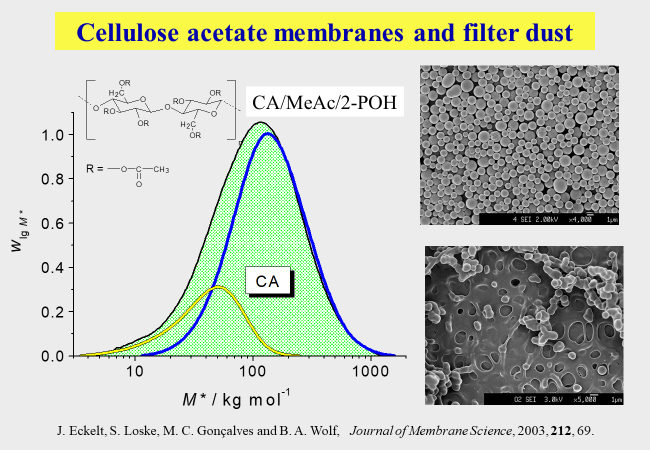
For medical applications of polymers, it is often necessary to remove harmful components, like in the case of hyaluronic acid, where too short chains can cause inflammation. How such a purification can be achieved is demonstrated in the references [^279] [^281]. Contaminants are also troubling industrial processes; one example is the preparation of membranes from cellulose acetate. In this case too short chains cause the formation of "filter dust" that must be brushed off from the final product. This problem can be solved by the use of fractionated cellulose acetate [^231].
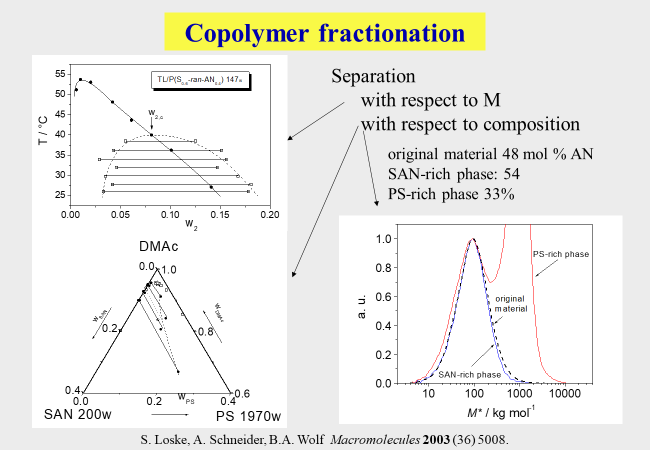
Due to the fact that the CSF process separates the components of polydisperse polymer products according to the differences in their solubility, the method can also be applied to fractionate copolymers with respect to their comonomer content[^223] [^246] [^276]. For that purpose the main task consists in finding components, which allow a thermodynamic discrimination of the two types of monomeric units. In the example shown below[^246] (fraction of random copolymer of styrene plus acrylonitrile) these components consisted in dimethylacetamide in combination with polystyrene. In contrast to the normal case the mixed solvent is not made up of two low molecular weight compounds, but of a solvent and a high molecular weight component.
In summing up it can be stated that CSF is applicable to all polymers that are prone to liquid/liquid phase separation. It yields economic access to large amounts of polymers with the uniformities in chain length or/and composition required for high-duty polymers in medicine and industry.
